Oxalic Acid Uses In Textile is industrial grade oxalic acid, the molecular formula is H2C2O4·2H2O, white crystal, mainly used in pharmaceutical, rare earth elements, smelting, fiber bleaching and other industries.
Property
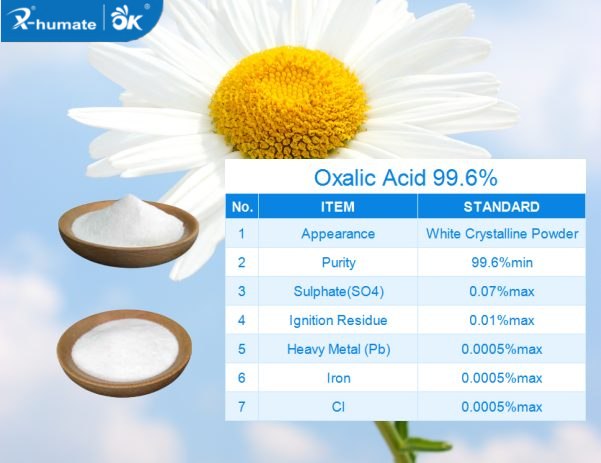
Oxalic Acid Uses In Textile is White transparent crystal combining two crystal water, melting point: 101-102oC, specific gravity:1.653,easily sublimate at 100oC, easily dissolve in alcohol and water.
Packing: In plastic woven bag with inner PE bag 25kgs net. White Bag or Grey bag
Capacity: 25MT/20FCL without pallet
Storage: Oxalic Acid Dihydrate should be stored in a cool, dry, well-ventilated area in tightly sealed containers.

Application:
Oxalic Acid Mainly used in pharmaceutical, rare earth elements, smelting, fiber bleaching and other industries.
Oxalic acid is a dicarboxylic acid of organic acids, and also one of the strong acids in organic acids.
Oxalic acid is also reductive, and oxidants are easy to be oxidized to carbon dioxide and water, and alkali neutralization, oxalate formation.
Oxalic ACID IS WIDELY USED IN THE chemical industry to produce penTAERYTHRITol, cobalt OXalate, nickel oxalate, alkaline green, steel, soil analysis reagents, chemical reagents and so on.
Oxalic Acid Uses In Textile also has two major applications in printing and dyeing.
It is used to wash and remove iron rust spots on fabrics Iron reacts with oxygen and water in the atmosphere to form complex compounds called rust.
The composition of rust varies with the conditions in which it is formed, but all rust contains ferric ions. The cotton fabric is stained with iron rust, which has formed defects and must be removed.
Oxalic acid is an effective agent for removing iron rust stains on cotton cloth.
The principle is that oxalic acid can form iron oxalate anionic chromium complex [F(C2O4)3] with the trivalent iron ion Fe+++ -- this anionic complex is easily soluble in water, so rust can be removed by oxalic acid washing.
Oxalic acid (20 g/L), acetic acid (98%) about 30 ml/L.
Oxalic acid is easy to damage the fiber, cotton after oxalic acid treatment, need to use water to thoroughly wash the oxalic acid remaining on the cotton fiber. If not washed, when the cotton cloth is dried, the dilute oxalic acid solution becomes concentrated acid, which will seriously damage the fiber quickly and cause holes, and attention must be paid to it.
The iron rust spots on the nylon fabric, will cause the fabric to yellow, can also be removed by oxalic acid.
Nitrous acid gas for the elimination of indycortin dyes
When USING INDYCORTIN DYE SODIUM NITRITE METHOD and NAFTOL dye together direct printing, through sulfuric acid when the production of nitrite, resulting in cloth NAFTOL AS sodium salt into a reddish brown precipitate, resulting in white.
In order to eliminate the nitrite, sometimes in sulfuric acid liquid color to add 1 ~ 2 grams/liter of oxalic acid instead of formic acid, urea and sulfur reductant, nitrite reduction of nitrogen, avoid HNO2 prompted AS brown.